Copper mobilisation from Cu sulphide minerals by methanobactin: Effect of pH, oxygen and natural organic matter
A new publication, co-authored by Danielle Rushworth (former guest of the ETH Soil Chemistry group), Iso Christl (ETH Soil Chemistry), Kevin Hoffmann (ETH Soil Chemistry alumnus), Ruben Kretzschmar (ETH Soil Chemistry) and colleagues from the Univeristy of Vienna, Univeristy of Wageningen and University of Basel, has recently been released online. The work, published in the journal Geomicrobiology, explores the mobilisation of Cu from Cu sulfide by methanobactin.
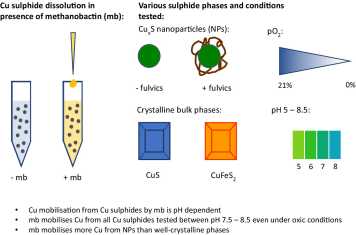
Aerobic methane oxidation (MOx) depends critically on the availability of copper (Cu) as a crucial component of the metal centre of particulate methane monooxygenase, one of the main enzymes involved in MOx. Some methanotrophs have developed Cu acquisition strategies, in which they exude Cu-binding ligands termed chalkophores under conditions of low Cu availability. A well-characterised chalkophore is methanobactin (mb), exuded by the microaerophilic methanotroph Methylosinus trichosporium OB3b. Aerobic methanotrophs generally reside close to environmental oxic–anoxic interfaces, where the formation of Cu sulphide phases can aggravate the limitation of bioavailable Cu due to their low solubility. The reactivity of chalkophores towards such Cu sulphide mineral phases has not yet been investigated. In this study, a combination of dissolution experiments and equilibrium modelling was used to examine the dissolution and solubility of bulk and nanoparticulate Cu sulphide minerals in the presence of mb as influenced by pH, oxygen and natural organic matter. In general, we show that mb is effective at increasing the dissolved Cu concentrations in the presence of a variety of Cu sulphide phases that may potentially limit Cu bioavailability. More Cu was mobilised per mole of mb from Cu sulphide nanoparticles compared with well-crystalline bulk covellite (CuS). In general, the efficacy of mb at mobilising Cu from Cu sulphides is pH-dependent. At lower pH, e.g. pH 5, mb was ineffective at solubilizing Cu. The presence of mb increased dissolved Cu concentrations between pH 7 and 8.5, where the solubility of all Cu sulphides is generally low, both in the pres-ence and absence of oxygen. These results suggest that chalkophore-promoted Cu mobilisation from sulphide phases is an effective extracellular mechanism for increasing dissolved Cu concentrations at oxic–anoxic interfaces, particularly in the neutral to slightly alkaline pH range. This suggests that aerobic methanotrophs may be able to fulfil their Cu requirements via the exudation of mb in natural environments where the bioavailability of Cu is constrained by very stable Cu sulphide phases.