New publication explores the 'interactions between iron and carbon in permafrost thaw ponds'
The article, recently published in Total Science of the Environment examines how iron influences carbon cycling in permafrost thaw ponds.
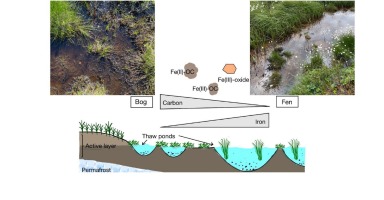
Thawing permafrost forms “thaw ponds” that accumulate and transport organic carbon (OC), redox-active iron (Fe), and other elements. Although Fe has been shown to act as a control on the microbial degradation of OC in permafrost soils, the role of iron in carbon cycling in thaw ponds remains poorly understood. Here, we investigated Fe-OC interactions in thaw ponds in partially and fully thawed soils (“bog” and “fen” thaw ponds, respectively) in a permafrost peatland complex in Abisko, Sweden, using size separation (large particulate fraction (LPF), small particulate fraction (SPF), and dissolved fraction (DF)), acid extractions, scanning electron microscopy (SEM), Fe K-edge X-ray absorption spectroscopy (XAS), and Fourier Transform Infrared (FTIR) spectroscopy. The bulk total Fe (total suspended Fe) in the bogs ranged from 135 mg/L (mean = 13 mg/L) whereas the fens exhibited higher total Fe (1.5 to 212 mg/L, mean = 30 mg/L). The concentration of bulk total OC (TOC) in the bog thaw ponds ranged from 50 to 352 mg/L (mean = 170 mg/L), higher than the TOC concentration in the fen thaw ponds (8.5 to 268 mg/L, mean = 17 mg/L). The concentration of 1 M HCl-extractable Fe in the bog ponds was slightly lower than that in the fens (93 ± 1.2 and 137 ± 3.5 mg/L Fe, respectively) with Fe predominantly (>75 %) in the DF in both thaw stages. Fe K-edge XAS analysis showed that while Fe(II) was the predominant species in LPF, Fe(III) was more abundant in the DF, indicating that the stage of thawing and particle size may control Fe redox state. Furthermore, Fe(II) and Fe(III) were partially complexed with natural organic matter (NOM, 8 to 80 %) in both thaw ponds. Results of our work suggest that Fe and OC released during permafrost thaw into thaw ponds (re-)associate, potentially protecting OC from microbial decomposition while also stabilizing the redox state of Fe.