Coprecipitation with Ferrihydrite Inhibits Mineralization of Glucuronic Acid in an Anoxic Soil
A new publication in the journal Environmental Science & Technology demonstrates the importance of mineral protection for the understanding of soil organic carbon mobilisation and degredation under reducing conditions.
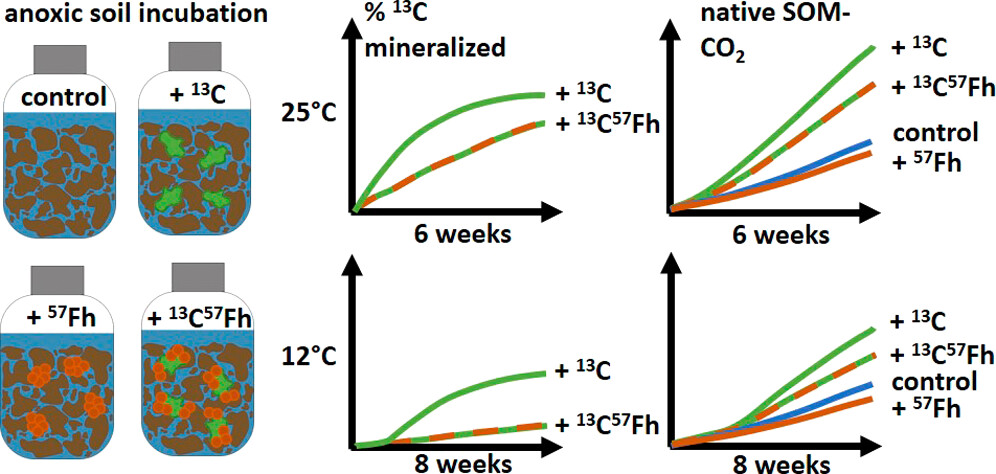
It is known that the association of soil organic matter (SOM) with iron minerals limits carbon mobilization and degradation in aerobic soils and sediments. However, the efficacy of iron mineral protection mechanisms under reducing soil conditions, where Fe(III)-bearing minerals may be used as terminal electron acceptors, is poorly understood. Here, we quantified the extent to which iron mineral protection inhibits mineralization of organic carbon in reduced soils by adding dissolved 13C-glucuronic acid, a 57Fe-ferrihydrite-13C-glucuronic acid coprecipitate, or pure 57Fe-ferrihydrite to anoxic soil slurries. In tracking the re-partitioning and transformation of 13C-glucuronic acid and native SOM, we find that coprecipitation suppresses mineralization of 13C-glucuronic acid by 56% after 2 weeks (at 25 °C) and decreases to 27% after 6 weeks, owing to ongoing reductive dissolution of the coprecipitated 57Fe-ferrihydrite. Addition of both dissolved and coprecipitated 13C-glucuronic acid resulted in increased native SOM mineralization, but the reduced bioavailability of the coprecipitated versus dissolved 13C-glucuronic acid decreased the priming effect by 35%. In contrast, the addition of pure 57Fe-ferrihydrite resulted in negligible changes in native SOM mineralization. Our results show that iron mineral protection mechanisms are relevant for understanding the mobilization and degradation of SOM under reducing soil conditions.
The external page full text is available online now.
ThomasArrigo, L. K., Vontobel, S., Notini, L. and Nydegger, T. (2023) Coprecipitation with Ferrihydrite Inhibits Mineralization of Glucuronic Acid in an Anoxic Soil. Environ. Sci. Technol. DOI: 10.1021/acs.est.3c01336