Coexisting Goethite Promotes Fe(II)-Catalyzed Transformation of Ferrihydrite to Goethite
Why do some product phases form preferentially to others during iron mineral transformation in soil? A new paper, published in Environmnetal Science & Technology by Luiza Notini (ETH Soil Chemistry), Laurel ThomasArrigo (ETH Soil Chemistry), Ralf Kaegi (EAWAG) and Ruben Kretzschmar (ETH Soil Chemistry), demonstrates that products of Fe(II)-catalysed transformation of ferrihydrite depend on the pre-existing mineralogy of the system.
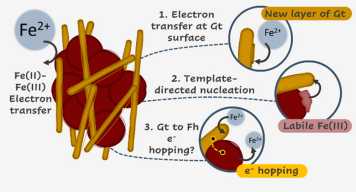
In redox-affected soil environments, electron transfer between aqueous Fe(II) and solid-phase Fe(III) catalyzes mineral transformation and recrystallization processes. While these processes have been studied extensively as independent systems, the coexistence of iron minerals is common in nature. Yet it remains unclear how coexisting goethite influences ferrihydrite transformation. Here, we reacted ferrihydrite and goethite mixtures with Fe(II) for 24 h. Our results demonstrate that with more goethite initially present in the mixture more ferrihydrite turned into goethite. We further used stable Fe isotopes to label different Fe pools and probed ferrihydrite transformation in the presence of goethite using 57Fe Mössbauer spectroscopy and changes in the isotopic composition of solid and aqueous phases. When ferrihydrite alone underwent Fe(II)-catalyzed transformation, Fe atoms initially in the aqueous phase mostly formed lepidocrocite, while those from ferrihydrite mostly formed goethite. When goethite was initially present, more goethite was formed from atoms initially in the aqueous phase, and nanogoethite formed from atoms initially in ferrihydrite. Our results suggest that coexisting goethite promotes formation of more goethite via Fe(II)–goethite electron transfer and template-directed nucleation and growth. We further hypothesize that electron transfer onto goethite followed by electron hopping onto ferrihydrite is another possible pathway to goethite formation. Our findings demonstrate that mineral transformation is strongly influenced by the composition of soil solid phases.
The external page full text is available online now.