New publication shows the effects of natural organic matter (NOM), metal-to-sulfide ratio and Mn(II) on cadmium sulfide nanoparticle growth and colloidal stability
Kevin Hoffmann (first author) has published the paper with Iso Christl, Ralf Kaegi (EAWAG) and Ruben Kretzschmar, investigating cadmium sulfide nanoparticle growth and stability. The work was published in Environmental Science: Nano.
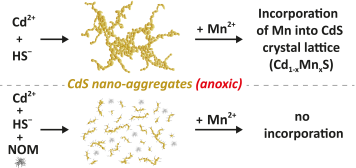
Redox-dynamic environments such as river floodplains and wetlands have been identified as sources of natural metal sulfide nanoparticles (MS NPs). However, little information is available on how their growth and colloidal stability are affected by the concentrations of metals and sulfide in solution, the presence of natural organic matter (NOM), and the possible incorporation of other metal cations such as Fe2+ or Mn2+. Here, we performed experiments on the formation of CdS nanoparticles (CdS NPs) in anoxic solutions with varying Cd (50, 100, 500 μmol L−1) and sulfide (50, 100, 1000 μmol L−1) concentrations in the absence and presence of Suwannee River fulvic acid (SRFA, 0, 5, 50 mg C per L). Additionally, we studied the influence of different metal-to-sulfide ratios and varying Mn2+ (0, 0.1, 0.5, 1 mmol L−1) concentrations on CdS aggregation using dynamic light scattering (DLS), transmission electron microscopy (TEM), and electrophoretic mobility measurements. The incorporation of Mn into the crystal lattice of CdS over 8 weeks was investigated with X-ray diffraction (XRD) and X-ray absorption spectroscopy (XAS). Our results show that after 24 hours, small CdS primary particles with median diameters of a few nanometers (TEM = 2–14 nm) formed large aggregates (TEM = 167 nm) and that increasing SRFA concentrations progressively constrained the size of these aggregates (down to 19 nm) irrespective of the initial reactant stoichiometry. When NOM was absent or at low concentration, higher metal-to-sulfide ratios (≥1) and Mn2+ concentrations (≥0.5 mmol L−1) led to reduced colloidal stability of the suspensions. We found that in suspensions containing Mn2+, 10–30% of the Cd atoms in the crystal lattice were substituted by Mn during the formation of CdS, which was prevented by NOM.
The external page full text is available online now.