New study investigates competitive incorporation of manganese and magnesium in vivianite
Vivianite, an iron phosphate mineral, commonly contains substitutions of manganese and magnesium. Until now, it has not been clear which substituent is preferentially incorporated into vivianite, or how salinity affects substitution. These questions have been tackled in a new study by Joëlle Kubeneck, Laurel ThomasArrigo, Katherine Rothwell and Ruben Kretzschmar from ETH Soil Chemistry, with Ralf Kaegi from EAWAG, which is now published in the journal Geochimica et Cosmochimica Acta.
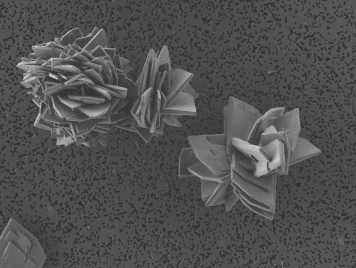
Vivianite, a ferrous phosphate mineral, can be an important phosphorus (P) sink in non-sulfidic, reducing coastal sediments. The Fe in the crystal structure of vivianite can be substituted by other divalent metal cations such as Mn2+ or Mg2+. Since Mg is much more abundant in coastal porewaters than Mn, the more frequent reports of Mn substitution in vivianites of coastal sediments has been suggested to indicate a preferential incorporation of Mn over Mg into the crystal structure of vivianite. However, although both Mn and Mg substitution in vivianite are environmentally relevant, it is yet unknown whether Mn or Mg is preferentially incorporated and how these isomorphic substitutions alter the crystal structure and morphology of vivianite, parameters which may influence vivianite reactivity. Here, we studied the incorporation of Mn and/or Mg in vivianites formed by co-precipitation at pH 7 in the presence of varying dissolved Mn and/or Mg concentrations and solution salinities resembling an estuarine gradient from 0 to 9 psu. In total, 19 different vivianites were synthesized, with up to 50% of Fe substituted by Mn and Mg. Thermodynamic equilibrium calculations showed that aqueous Mg speciation was altered with increasing salinity, while Mn speciation was less affected, likely explaining the preferential incorporation of Mn in the vivianite structure at higher salinities. 57Fe-Mössbauer spectroscopy revealed that both Mn and Mg were preferentially incorporated in the double-octahedral Fe position, at which intervalence charge transfer is possible during the oxidation of vivianite. In contrast to Mg, which is redox inactive, incorporated Mn can participate in heteronuclear intervalence charge transfer with Fe. Thus, incorporation of either cation may impact the reactivity of vivianite under oxidizing conditions in element specific ways. Results of complementary analyses including X-ray diffraction, electron microscopy and Fe K-edge X-ray absorption spectroscopy further showed that incorporation of Mn and/or Mg led to smaller particle size, increased crystal roughness and thinner crystals, as well as systematic changes in unit cell parameters. These observed changes in crystal morphology might impact the reactivity of vivianite in natural environments and thus the effect of cation incorporation in vivianite should be considered when studying Fe and P cycling in coastal sediments.